Turning bad fat
into good fat
The identification of a protein which is critical to turning white fat to brown fat has implications for the treatment of metabolic diseases.



![]() We are seeking to understand the regulatory process, which will give further insight into dealing with metabolic
We are seeking to understand the regulatory process, which will give further insight into dealing with metabolic
diseases – and not only these but there are implications for heart diseases and some cancers. ![]()
Dr Roger Wong Hoi-fung
When it comes to the battle with weight gain, or regulating metabolic imbalance, white fat is the enemy, and brown fat the friend. In adults 90 per cent of body fat consists of white fat, which stores excess energy and leads to weight increase. Brown fat in adults makes up 10 per cent of body fat and when activated can reduce body weight.
“Until recently, scientists thought only infants had brown fat, but now we have discovered that if stimulated by cold, we can actually activate brown fat in adults,” said Dr Roger Wong Hoi-fung, Research Assistant Professor in the Department of Chemistry, who has been working with a research team at the University of California, Berkeley, to identify a new protein critical for the conversion of white fat to brown fat.
“Brown fat is a type of ‘good fat’ if you like,” said Dr Wong. “It burns calories, rather than storing them like white fat, and in the process produces heat.”
The research team’s aim was to utilise the advantages of brown fat by searching for ways to enable the body to preserve, increase and activate brown fat, with the ultimate aim of developing a tool to fight obesity, type 2 diabetes, non-alcoholic fatty liver disease and other metabolic diseases.
“We needed to identify a fat-burning gene which can trigger the process of ‘browning’ the white fat which stores excess energy in the body to enable us to resist cold and lose weight,” said Dr Wong. “We were looking for the transcription factor that controls either the formation of brown fat or how white fat can be turned into brown fat. It was like seeking one particular fish in an ocean. In humans there are 30,000 genes.
“There were two possible strategies: one, look at those 30,000 genes individually one by one and see which one could activate brown fat gene (UCPI); and two, use a super computer to do the bioinfomatics. In the end, both approaches produced the same result,” said Dr Wong. “Each identified the protein Zfp516 as the earliest driving force in the formation of brown fat, and discovered that levels of this protein increase during exposure to cold temperatures.”
In experiments where transgenic mice were fed on a high-fat diet, those with higher levels of the Zfp516 protein were found to gain 30 per cent less weight than their counterparts. In addition, extended exposure to cold temperatures (4 degrees Celsius) also triggered the protein. This study has been published in the journal Molecular Cell.
By activating the protein Zfp516 through potential drugs in the future it may be possible to promote weight loss. Berberine, a natural product used in traditional Chinese medicine may activate Zfp516. “It was known previously that Berberine might activate a browning of white fat,” said Dr Wong, “but we didn’t know the mechanism involved. Our research has now explained that.”

The active ingredient in Chinese herbs with Berberine is noted to have the effect of triggering the ‘browning’ of white fat. Dr Wong's research team looks into its relation with Zfp516 protein.
Regulating metabolism
The next step is to continue to identify genes involved in both the generation of white fat and the generation of brown fat and to work out if it is possible to electively impact the regulation of fat metabolism.
“We are seeking to understand the regulatory process, which will give further insight into dealing with metabolic diseases – and not only these but there are implications for heart diseases and some cancers. Further, information generated by lipodaemia – which is termed metabolism information – may be converted into neuro-information and give us insights into Alzheimers and other degenerative neuron diseases.
“For example, we know that people who have type 2 diabetes have a higher risk in less than 10 years of developing Alzheimers or Parkinson’s disease. It is a long process and no one knows what happens in-between. If we can identify what’s in-between – that is, the trigger that sparks Alzheimers or Parkinson’s – may be we can do something about it,“ said Dr Wong.
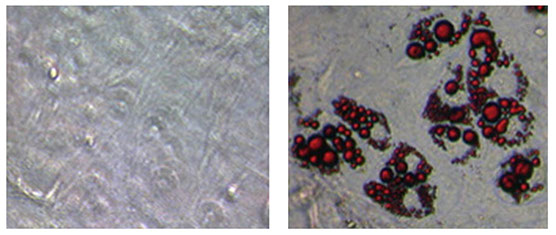
The fat-burning gene Zfp516 protein triggers ‘browning’ of white fat. The Oil Red O staining in the above photo indicates the presence of brown fat.

