DATA WIZARD ADVANCES PRECISION MEDICINE
Ten years ago, it took a month to map the genome of a person using a huge computational server. Now this can be done in less than a day with a desktop computer, thanks to up-and-coming scientist Dr Luo Ruibang, who has also led the way in applying big data and artificial intelligence to genetic analysis.
Next
Precision medicine is the new goal in medicine, as doctors seek to harness genetic information to provide patients with more effective diagnoses and treatments. But a key stumbling block has been the enormous quantity of this information. Each person’s chromosomes contain more than three billion characters. Until the work of Dr Luo Ruibang, sifting through that information was a laborious process.
Dr Luo developed a new algorithm that greatly speeds up the process by quickly matching groups of characters called ‘reads’ to their chromosomes, so doctors and scientists can more readily detect mutations. Whereas previously it took one month to do this using a large powerful computer, at a cost of about HK$100,000, Dr Luo’s algorithm was able to reduce the time to less than a day using a laptop.
The algorithm was developed with his former teacher and the current head of Computer Science at HKU, Professor Lam Tak-wah, in 2009 when Dr Luo was only 20 years old. It is now a global standard for genome alignment, having been downloaded more than 300,000 times.
“It was not rocket science but there was a ‘eureka’ moment,” he said. “We were able to lower the complexity of the previous algorithm to reduce the time it takes to align the reads back to the chromosomes. That’s important for patients because they are awaiting their results.”
The discovery was a first step in a career that, to date, has seen Dr Luo named by MIT Technology Review as one of the top 10 innovators in Asia Pacific in 2019, and by Forbes as one of the top ‘30 Under 30’ in Asia in 2017 in healthcare and science.
Platform launches a start-up
The next step was to apply big data analytics to provide better and faster interpretations of whether the genetic mutations detected by his algorithm are pathogenic. This requires comparing a patient’s mutations with multiple databases that do not completely agree with each other.
Previously, doctors had to cut and paste their patient’s mutations into a search window for each database and write or type out the results manually for comparison, a process that took five hours per patient and was typically limited to about 10 databases. Dr Luo and his team vastly improved the process.
After working with doctors for two years to understand how they used the databases, Dr Luo developed a platform that can search 31 databases within one hour – two hours in a worst-case scenario. Most importantly, it includes sophisticated rules for deciding when there is a conflict in the results from the search and how to present this to doctors.
The platform was developed into a highly-successful start-up, L3 Bioinformatics Ltd, that was launched in 2014 with support from the Hong Kong Government, angel investor Beijing Genomics Institute and HKU’s Technology Transfer Office, and has generated more than HK$60 million in revenue. The platform has been used to diagnose more than 5,000 cancers and rare diseases at the Hong Kong Sanatorium Hospital and Hong Kong’s Department of Health, as well as thousands more cases in Mainland China.
Dr Luo worked with L3 Bioinformatics for two years but returned to academia in 2016 because there were still important research questions to tackle – particularly, how to improve the matching rate of DNA mutations to databases. No technology has achieved a better actionable rate than 30 per cent which means no diagnostic or therapeutic match can be found for genetic mutations in 70 per cent of cases.
Pulling signals from the noise
“There is still a lack of knowledge about human genomics – we are dealing with three billion characters. I hope to boost the matching rate to 40 or 50 per cent in future, leveraging artificial intelligence,” he said.
Already, he has applied machine learning to reduce the number of errors in reads, which is especially problematic for smaller DNA readers. “I’m the first to directly pull out the useful mutation signals from the noise in single-molecule sequencing [also known as third-generation sequencing],” he said. This opens the possibility of doctors being able to pipe a patient’s DNA sample into a USB-sized device and get results within a couple of hours.
This discovery has also been found to be efficient at detecting pathogens, such as viruses, and he is working with Johns Hopkins University to develop that avenue further.
Dr Luo is keen to do more technology transfer and stay at the frontier of science. He hopes more young people will join him. “I was not a typical top-student but after all these years, I find teaching students is fun, especially about cutting-edge technologies that they can’t find in textbooks. Hong Kong lacks experts in precision medicine and bioinformatics so one of my ambitions is to raise some good experts in this field for Hong Kong and globally.”

![]() There is still a lack of knowledge about human genomics – we are dealing with three billion characters. I hope to boost the matching rate to 40 or 50 per cent in future, leveraging artificial intelligence.
There is still a lack of knowledge about human genomics – we are dealing with three billion characters. I hope to boost the matching rate to 40 or 50 per cent in future, leveraging artificial intelligence. ![]()
Dr Luo Ruibang

Dr Luo uses sequencing technologies to shorten the time required for cancer and rare disease diagnosis.
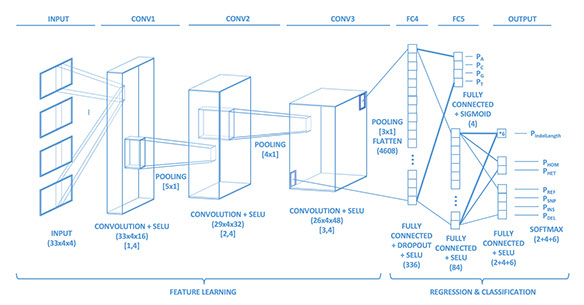
The deep neural network architecture published in the paper named ‘Clairvoyante’ for extracting weak variant signals from very noisy single molecule sequencing data.
The miniaturised MinION® single molecule
sequencing device used in the paper for reading out DNA.

Home
May 2019
Volume 20
No. 2

Research
