Tales From The Flu Front
The H7N9 influenza virus has the potential to become a pandemic threat. If this does not happen, then Professor Guan Yi and his team may be the ones to thank.
How does one contain a pandemic? For Professor Guan Yi, Daniel CK Yu Professor in Virology, it comes down to old-fashioned legwork. He and his team have collected thousands and thousands of influenza samples over the past 15 years from the breeding and transmission grounds of new and old viruses – chickens, ducks and other birds found in markets and farms in China and patients hospitalised with mysterious flu-like illnesses – to track their progress and raise alarms.
The painstaking and persistent work has made Professor Guan one of the top microbiologists in the world. But it has not diminished his biggest problem: not the birds, not the viruses, but the human tendency to brush aside warnings that may be inconvenient.
His recent work on H7N9, a particularly lethal virus which has killed more than one-third of the people it has infected, is no exception.
Only weeks after China reported the first infected patient in spring, 2013, Professor Guan and his team – which included scientists from Shantou University and the United States and Britain – reported in both Nature and Science how and where the virus had emerged and showed conclusively that the main source was chickens. “We told people directly how it evolved step by step by transmitting from migratory bird to domestic duck, then getting into chicken, and finally re-assorting with the chicken H9N2 influenza viruses to generate this H7N9 virus,” he said.
HKU’s School of Public Health and others began to raise the alarm, but not everyone was convinced. Poultry markets, believed to be a main incubator of the virus, remained open in Mainland China. And over the next couple of years, in three successive waves of H7N9 infections, an anomaly was reported by Mainland authorities: infections attributed to human-to-human transmission increased, but those from chickens dropped to zero.
“This was impossible!” Professor Guan said. “The source of the human virus is actually an animal virus. If we want to control this outbreak, control this source, we have to get rid of the infected chickens.”
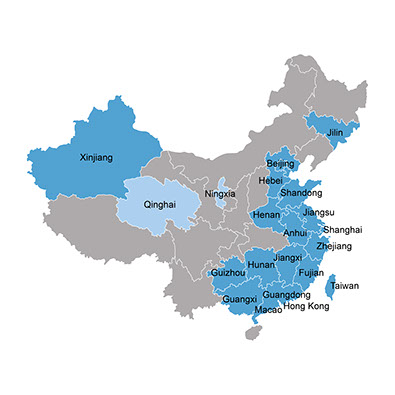
The above map shows the H7N9 distribution across China, as reported in the research. Deep blue indicates provinces with human infection cases. Light blue indicates provinces with H7N9 viruses reported in poultry or environmental samples at the live poultry markets.
Distribution of the reported H7N9 infection cases
with H7N9 human cases
with H7N9 in birds
without reported cases

![]() I don’t know whether we still have a chance to eradicate the virus in the field, but as a professor at HKU, I have a duty to educate not just our students but the whole world about the options, so they can decide how to make people more safe.
I don’t know whether we still have a chance to eradicate the virus in the field, but as a professor at HKU, I have a duty to educate not just our students but the whole world about the options, so they can decide how to make people more safe. ![]()
Professor Guan Yi
Lethal consequences
As if to underscore the point, the number of people infected with H7N9 continued to increase. As of mid-March this year, 638 people had been infected and 229 of them had died (Professor Guan said more people were certainly infected because it was unlikely every case was tested or reported). Moreover, the infections had spread from East China, where the virus was first reported, to Guangdong and other provinces.
Professor Guan was sufficiently alarmed by this growing public health threat that he got his team to work even more intensively on H7N9, and again linked up with Shantou University as well as Shenzhen Third People’s Hospital, Zhejiang Provincial Center for Disease Control and Prevention, and American and Australian scientists.
They tested samples collected from 16,299 chickens in more than 15 cities in five provinces in China, and showed conclusively that the virus was present in three to five per cent of the birds (15 per cent at peak time), and that the number of variants found in chickens that could infect humans was increasing.
The scientists said the persistence and diversification of the virus was likely due to its spread along poultry trade routes. They also reported that they had detected other viruses with potential to infect humans, including H7N7, H7N6 and H10N6.

Professor Guan Yi (centre in the front row), Daniel CK Yu Professor in Virology, and his team.
Renewed warning
The results were published this spring in Nature and Science, with the warning that unless effective control measures were in place, the virus would continue to spread.
“If a virus has more time to cook, then the whole population may get much more seriously infected,” Professor Guan said.
Fortunately, the renewed warning has fallen on the right ears. Soon after the recent research was published, the Guangdong Government announced that major cities in the Pearl River Delta would close their live poultry markets with immediate effect and all other cities would close them by October
this year.
“I don’t know whether we still have a chance to eradicate the virus in the field, but as a professor at HKU, I have a duty to educate not just our students but the whole world about the options, so they can decide how to make people more safe.”
Ironically, Professor Guan was due to present his findings in person at a conference in Seoul in June but had to appear via Skype instead due to an outbreak of Middle East Respiratory Syndrome. Similar to H7N9, people were seemingly indifferent to the dangers and continued to travel and even ignore quarantine orders. “This is much more difficult than birds!” he said.



