|
|
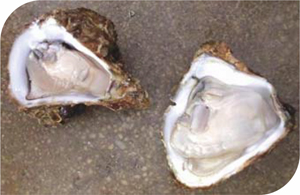
The future of the global shellfish industry could be in jeopardy if carbon dioxide (CO2) emissions are not curtailed. Research underway in the Swire Institute of Marine Science and School of Biological Sciences suggests that rising CO2 in coastal waters can have a devastating effect on marine species, particularly shellfish. Dr V. Thiyagarajan (Rajan), who has been studying Hong Kong's native oyster population, explains that high-CO2 affects the pH balance of the oceans changing its carbonate chemistry, the process popularly called as ocean acidification. "Currently there is around 380ppm of CO2 in the atmosphere," he says. "We expect that to double in the next 100 years. The ocean absorbs about 70% of this CO2. So the majority of it goes into the ocean and coastal waters. In seawater, CO2 combines to form bicarbonate iron. The higher the CO2 content the higher the bicarbonate iron. This, in turn, lowers the levels of carbonate iron, without which marine species cannot develop their healthy shells," says Dr Rajan. A century ago the pH of the seawater stood at 8.2. Now it is 8.1 globally, indicating that the system has already been disturbed. Without intervention the pH is expected to plummet to 7.6 over the next 100 years. Key ingredients for shells "This means the carbonate will be reduced 300-fold. And without sufficient carbonate the majority of shellfish are unable to make their shells. This will affect the abundance of shellfish in the sea and also the shellfish industry. If we lose these shellfish it will have a huge impact because they are part of our coastal fishery resources and they play a key role in maintaining the coastal eco-system." Dr Rajan is studying the larvae of the edible oyster species to see how well they perform under different emerging stress conditions including ocean acidification. |
"We have been experiencing three emerging environmental stressors," he says. "One is the CO2, if you raise it, it changes the carbonate chemistry as we've just discussed; on top that in Hong Kong we are expecting heavy rain in the next 100 years because of global climate change. The southern part of China will get a lot of heavy rainfall, then the amount of fresh water flowing down from the Pearl River will reduce the salinity. So we will have high-CO2, low salinity and high temperatures. We are mimicking these conditions in the lab and exposing the larvae to them." |
|
What he has found is that when the carbonate level drops the larvae try to increase the pH level in the vicinity. "Although they are tiny they have the mechanism to do this," he says. "This is how they have survived for millions of years. So they are trying to adapt to their environment and they have been doing that very well so far. But we are putting them under pressure and seeing how much they can tolerate. Externally they change and we are looking at how much they can change and whether they can achieve it or not, while internally we see three things happening. Firstly, making the shell is a problem, if they can't make the shell they can't go to the next stage. Predators will catch them. Secondly, the shell's mechanical properties, the hardness of the shell, is governed by unique shell-making proteins but they are unable to synthesise these specialized proteins anymore because they are under stress. Thirdly, if they have a more fragile shell what will happen to them, will they reach maturity and if they do how long can they sustain life?" Dr Rajan's large-scale and long-term controlled experiment in a commercial hatchery setting showed that larval shell growth rate is significantly reduced at projected carbonate chemistry scenarios in 2100 in the oyster species. This industry supports the livelihoods of millions of people in South China. The death of a delicacy Dr Rajan expects the early life stages of oysters, green mussel and sea snails to be the most affected by changes in ocean acidification because low-pH water is highly corrosive to their fragile shells made of aragonite. "What's happening now in the southern coastal waters, like the Polar waters, is that species that use aragonite to make their shells are going to be severely affected. So no more New Zealand mussels and oysters. Scientists are expecting very dramatic problems in the next 50 years. In Hong Kong we are quite safe at the moment because our pH balance is not being affected that much because our warmer sub-tropical waters means the CO2 is dissipated faster. But it will get worse unless we do something about it." With a grant of $4 million from the UGC, RGC and HKU he is working with oyster farmers in Lau Fu Shan village, a traditional oyster village, to see how resilient the native Hong Kong species is. "Internationally this is a huge topic and billions of dollars are being ploughed into this type of research, but I am the only one conducting it in Hong Kong. This is a very, very serious problem in coastal ecosystems." But it's not all bad news. Dr Rajan says there are ways to solve the problem. "Coastal habitats such as wetlands soak up CO2 from the atmosphere and bury it in the sediment. If we manage our coastal habitats and wetlands, for example, if we have one wetland for every power plant then we can manage CO2 emissions. "Alternatively, many plant species (sea grass, phytoplankton and sea weeds) in coastal waters absorb CO2. If we increase the number of plants then it's possible that they will absorb more CO2 and thus stabilize the reaction. So there are scientific solutions."
|
| Back | Next | |