Dr Tan-Un Kian Cheng from HKU’s School of Biological Sciences led the team which made the discovery, working in collaboration with Professor Richard Festenstein at the Clinical Sciences Centre, Imperial College London, Professor Sjaak Philipsen at Erasmus University MC, Rotterdam and Professor Godfrey CF Chan, Tsao Yen-Chow Professor in Paediatrics and Adolescent Medicine at Department of Paediatrics and Adolescent Medicine, HKU. Their findings were published in the journal Nucleic Acids Research.
Said Dr Tan-Un: “Finding the part of the human genetic machinery which switches on the Neuroglobin gene was a thrilling moment, and of paramount importance. It opens up opportunities such as paving the way for developing novel therapeutic treatments using gene therapy or drugs to target Alzheimer’s. The global population is ageing and this disease is affecting larger and larger numbers – currently it is incurable, so this discovery is critical.
“Research on mice has shown that Neuroglobin protects the brain and can reduce the severity of damage due to stroke and Alzheimer’s. In humans, the two major risk factors for chances of getting Alzheimer’s are age and being female, both of which are associated with lower levels of Neuroglobin.”
Neuroglobin was first identified in 2000, but its function was unknown. In 2005, the link between Neuroglobin and Alzheimer’s was made when it was discovered that the gene’s presence prevents the formation of amyloid plaques, which is a hallmark of Alzheimer’s.
Dr Tan-Un’s team spent six years looking into the mechanism that controls the switching of the Neuroglobin gene. It was a long and technically-challenging search. “Neuroglobin was a new discovery, and my group was the first to characterise the gene,” she said. “We were asking questions such as how it would be regulated and what sequence could control the expression of Neuroglobin? Previous studies have established that distal elements are important in regulating cell-specific gene transcription and mutations in these distal elements are known to cause diseases such as thalassaemia. We therefore hypothesised that the novel elements located far beyond the Neuroglobin gene may be potential regulatory elements.”
Her former PhD students Zhang Wei and Tam Kin-tung started working on the technically-challenging problem of searching for the distal DNA region. They carried out many experiments, including using a technique called chromosome conformation capture (3C). “Zhang Wei identified the distal regulatory elements within two months,” said Dr Tan-Un. “Tam Kin-tung then started working on it, asking questions such as whether the distal element controls Neuroglobin gene expression, and what transcription factors bind to this region?”
They narrowed down the region through further experimentation, and the breakthrough came when the team discovered a segment of DNA which interacts with the Neuroglobin gene by means of a protein called GATA-2 in human neuronal cells. The novel DNA segment proved to be powerful in switching on Neuroglobin expression. They further discovered that removing either the GATA-2 protein or the DNA segment from the cells led to a substantial decrease in Neuroglobin expression.
![]() Finding the part of the human genetic machinery which switches on the
Finding the part of the human genetic machinery which switches on the
Neuroglobin gene was a thrilling moment,
and of paramount importance. It opens up opportunities such as paving the way for developing novel therapeutic treatments
using gene therapy or drugs to target Alzheimer’s. ![]()
Dr Tan-Un Kian Cheng
Passion to discover
“Research is all about passion to discover,” said Dr Tan-Un, “but at times we were very down – a lot of tedious work for little progress. But it was good to be working in collaboration with our colleagues from Imperial and Erasmus as we could do Skype conferencing to brainstorm problems. It was beneficial for keeping our spirits up.”
Having discovered the ‘switch’, Dr Tan-Un said that gaining a deeper understanding of both the switch and the Neuroglobin locus is now critical in designing an efficient gene therapy system for the treatment of Alzheimer’s and other neurodegenerative diseases.
Since increased Neuroglobin expression helps protect against Alzheimer’s, the next stage of the research will focus on identifying the factors that will enhance the ‘switch’ mechanism in the brain. “We will collaborate with Professor Godfrey CF Chan from HKU’s Faculty of Medicine and Professor Richard Festenstein at Imperial College on the next stage,” she said.
Dr Tan-Un added that the research is a continuous endeavour, pointing out that her own association with this area of biological science is long-standing: her PhD study was on the identification of mutations in the beta globin gene and elucidating the molecular pathology of the mutations that caused the disease thalassaemia. The groundbreaking discovery of a distal locus control region (LCR) was made in 1987 by her former PhD mentor at the National Institute for Medical Research at Mill Hill in London, Professor Frank Grosveld [currently at the Department of Cell Biology at Erasmus University MC, Rotterdam]. “And, now that PhD students I am mentoring are making discoveries in this area, it feels like the connections are being made, so the search for distal regulatory elements or LCRs continues as the knowledge passes down from generation to generation,” she said.
The working team from the School of Biological Sciences — (from left) Dr Gigi So, Dr Annie Law, Dr Tan-Un Kian Cheng, Mr SK Chan, Dr Tian Zhipeng and Dr Tam Kin-tung.
Dr Tan-Un Kian Cheng and her team’s findings were published in the journal Nucleic Acids Research.


Flicking
the right switch
A multinational team of biological scientists has opened the door to new possibilities for fighting Alzheimer’s by discovering the switch for Neuroglobin, a gene known to play a role in protecting the human brain from neurodegenerative diseases.
Next
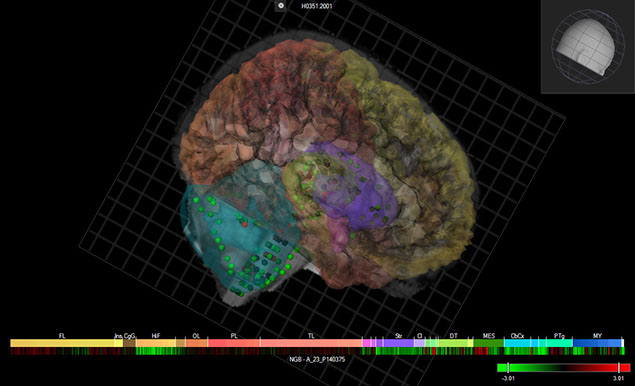
Neuroglobin expression in the human brain.(Courtesy of Allen Human Brain Atlas)


